From ethylzinc guanidinate to [Zn10O4] supertetrahedron
Michał K. Leszczyński,a Iwona Justyniak,a Karolina Zelga,b Janusz Lewiński,ab
a Institute of Physical Chemistry, Polish Academy of Sciences, Kasprzaka 44/52, 01-224 Warsaw, Poland
b Faculty of Chemistry, Warsaw University of Technology, Noakowskiego 3, 00-664 Warsaw, Poland
DOI: 10.1039/C7DT01619K
First published online 14 Jun 2017.
Paper at Publisher's website
Cover!
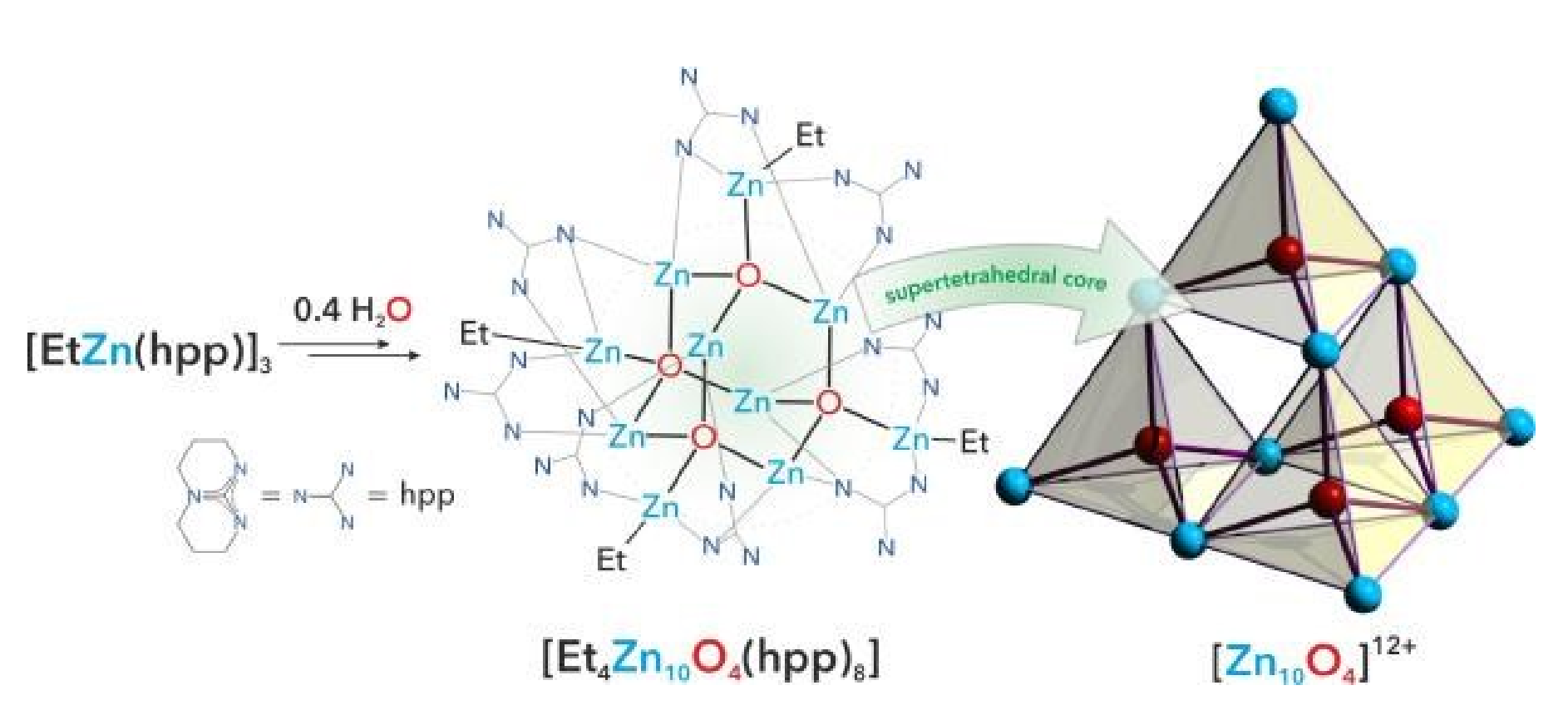
The controlled hydrolysis of an ethylzinc guanidinate complex which affords the alkylzinc cluster containing a [Zn10O4]12+ supertetrahedron core stabilized by the guanidinate ligands is described. Accompaning investigations on the reactivity of this unprecedented cluster toward alcohols resulted in the formation of a mononuclear zinc alkoxide supported by the guanidinate ligands.
and
Toward Factors Affecting the Degree of Zinc Alkyls Oxygenation: A Case of Organozinc Guanidinate Complexes
Michał K. Leszczyński,a Iwona Justyniak,a Janusz Lewiński,ab
a Institute of Physical Chemistry, Polish Academy of Sciences, Kasprzaka 44/52, 01-224 Warsaw, Poland
b Faculty of Chemistry, Warsaw University of Technology, Noakowskiego 3, 00-664 Warsaw, Poland
DOI: 10.1021/acs.organomet.7b00287
First published online 19 Jun 2017.
Paper at Publisher's website
While extensive research has been carried out on the oxygenation of alkylzinc complexes for decades, this issue still remains unresolved and significant uncertainties concerning the mechanism of these reactions and the composition of the resulting products persist. These reactions are believed to proceed via the initial formation of ROOZn(L) species, but studies on the oxygenation reactions have been substantially impeded by the low stability of the alkylperoxides. This report describes the oxygenation of a tert-butylzinc guanidinate, i.e. a tBuZn(L)-type complex (L = deprotonated 1,4,6-triazabicyclo[3.3.0]oct-4-ene), and the isolation and characterization of an unprecedented aggregate based on a combination of a ROOZn(L) moiety and two parent tBuZn(L) molecules, {[tBuZn(L)]2[tBuOOZn(L)]}. Further study revealed that examined tert-butylzinc guanidinate molecules exhibit an ability to entrap other oxygenated species, which was demonstrated by an aggregate containing a tert-butylzinc tert-butylperoxide and two tBuZn(L) molecules, {[tBuZn(L)]2[tBuOZntBu]}. Thus, the reported studies indicate that entrapment of the product of an oxygenation reaction by the parrent alkylzinc species is another important factor controlling the oxygenation of organometallics.
