Unprecedented variety of outcomes in the oxygenation of dinuclear alkylzinc derivatives of a N,N-coupled bis(β-diketimine)
Tomasz Pietrzak,a Maciej Korzyński,b Iwona Justyniak,a Karolina Zelga,a Arkadiusz Kornowicz,a Zbigniew Ochal,a Janusz Lewiński,ab
a Faculty of Chemistry, Warsaw University of Technology, Noakowskiego 3, 00-664 Warsaw, Poland
b Institute of Physical Chemistry, Polish Academy of Sciences, Kasprzaka 44/52, 01-224 Warsaw, Poland
DOI: 10.1002/chem.201700503
First published online 11 Apr 2017.
Paper at Publisher's website
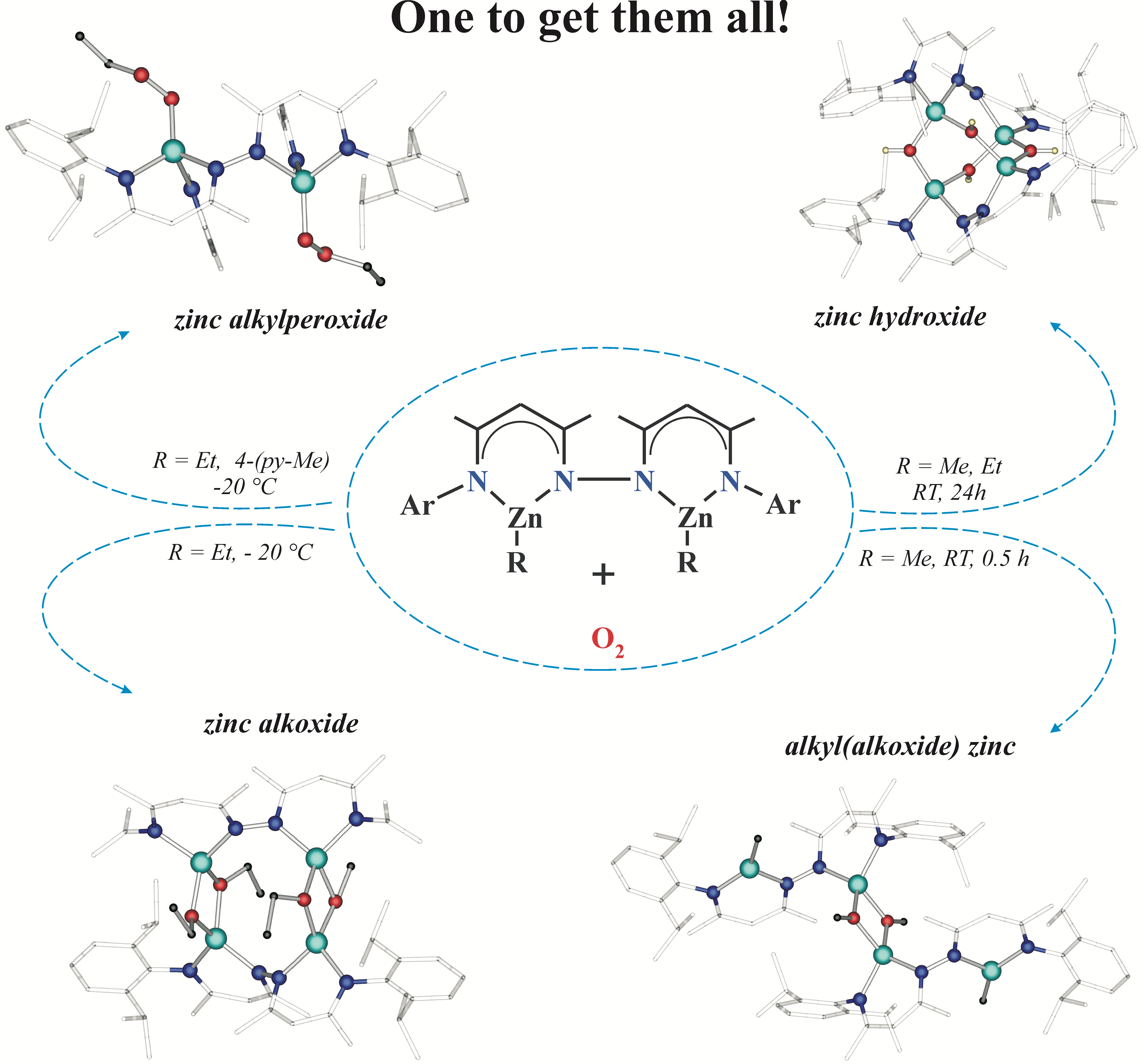
Reactions between O2 and organometallics with non-redox active metals center have received continuous interest for over 150 years, although significant uncertainties concerning the character and details constituting the actual mechanism of these reactions persist. Harnessing dinuclear three-coordinate alkylzinc derivatives of a N,N-coupled bis(β-diketimine) proligand (L-H2) as a model system, we demonstrate for the first time that a slight modification in the reaction conditions might have a dramatic influence on the oxygenation reaction outcomes leading to unprecedented variety of products originating from a single reaction system, i.e. partly and fully oxygenated zinc alkoxides and zinc alkylperoxide and hydroxide compounds. Our studies indicate that accessibility of the three coordinate zinc center for O2 molecule coupled with the lower reactivity of Zn-Me vs Zn-Et units towards dioxygen are key factors in the oxygenation process, providing a novel tetranuclear methyl(methoxy)zinc {[L][ZnMe][Zn(μ-OMe]}2 and zinc ethoxide {[L][Zn(μ-OEt)]2}2. Remarkably, the oxygenation of three-coordinate alkylzinc [L][ZnR]2 complexes at ambient temperature afforded a unique hydroxide {[L][Zn(μ-OH)]2}2. The oxygenation of [L][ZnEt]2 complex in the presence of 4-methylpyridine (py-Me) at low temperature led to the isolation of a dinuclear zinc ethylperoxide [L][Zn(OOEt)(py-Me)]2, which nicely substantiates the intermediacy of an unstable zinc alkylperoxide in the formation of the subsequent zinc alkoxide and hydroxide compounds. Finally, our investigations provide compelling evidences that non-redox-active metal center plays crucial role in the oxygenation process through assisting in single electron transfer from a M-C bond to a O2 molecule. While the oxygenation reaction of zinc alkyls occurs by radical pathways, the reported results stand in clear contradiction to the widely accepted free-radical chain mechanism.