Marcin Kubisiak,a Karolina Zelga,a Wojciech Bury,a Iwona Justyniak,b Krzysztof Budny,a Zbigniew Ochala and Janusz Lewiński*ab
a Faculty of Chemistry, Warsaw University of Technology, Noakowskiego 3, 00-664 Warsaw, Poland
b Institute of Physical Chemistry, Polish Academy of Sciences, Kasprzaka 44/52, 01-224 Warsaw, Poland
DOI: 10.1039/C5SC00600G.
Received 16 Feb 2014, Accepted 19 Mar 2015
First published online 19 Mar 2015
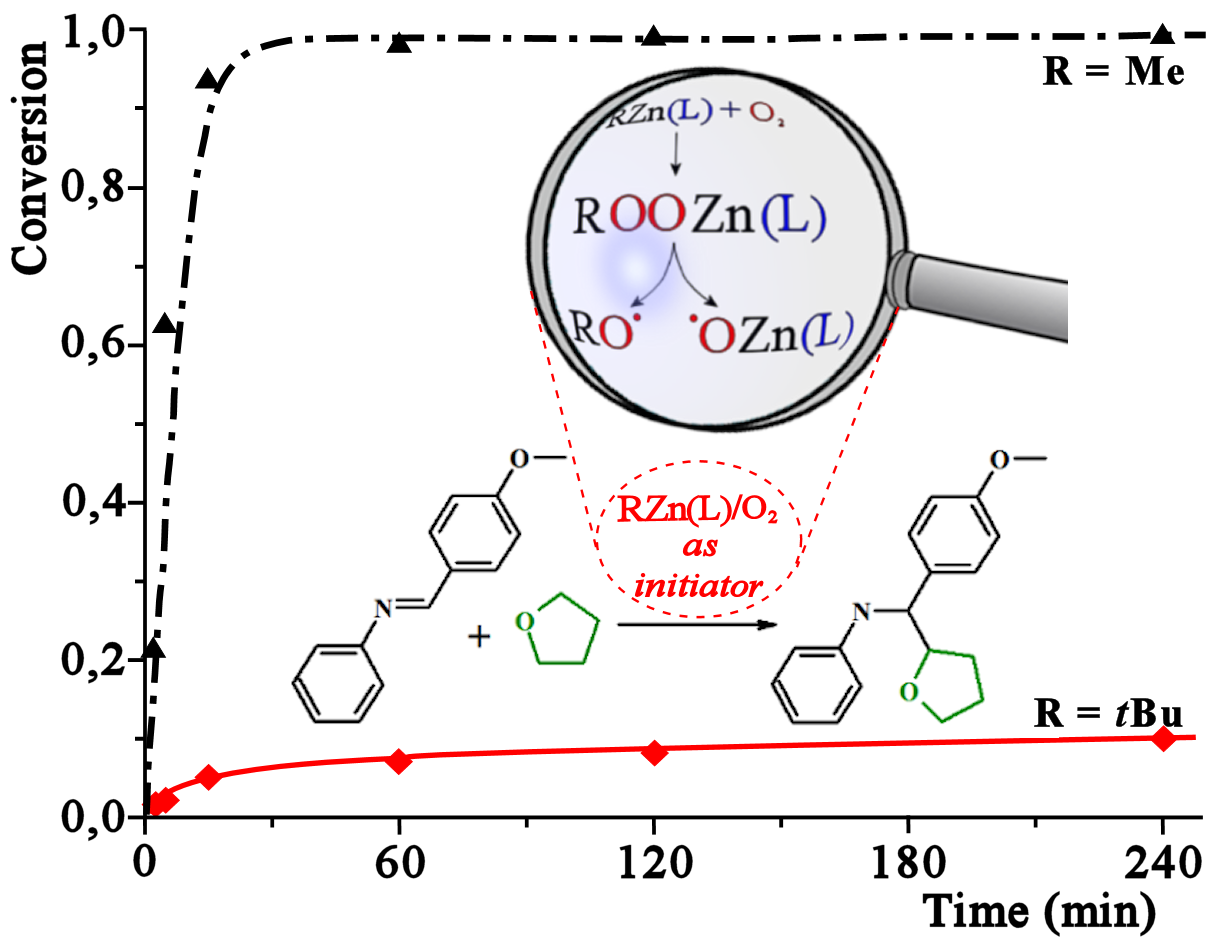
This paper reports a series of comparative experiments on the activity of carbon- and oxygen-centred radical species in a model reaction of radical addition of THF to imines mediated by a series of zinc alkyl/O2 reaction systems. The study strongly contradicts the notion that generally R• radicals are the initiating species in organic reactions mediated by RnM/O2 systems and simultaneously demonstrates that oxygen-centred radical species are the key intermediates responsible for the initiation process. In addition, a new efficient RZn(L)/O2 initiating system for radical organic reactions exampled by a model reaction of radical addition of THF to imines is developed. Moreover, the isolation and structure characterization of the first zinc alkylperoxide supported by a carboxylate ligand, [Zn4(μ3-OOtBu)3(μ4-O)(O2CEt)3]2, as well as the novel octanuclear zinc oxo(alkoxide) aggregate with entrapped O–THF species, [Zn4(μ4-O)(μ3-2-O–THF)(O2CEt)5]2, provide clear mechanistic signatures for the mode of the RZn(O2CR’)/O2 system’s function.