Krzysztof Budny-Godlewski,a Dominik Kubicki,a Iwona Justyniakb and Janusz Lewiński*ab
a Faculty of Chemistry, Warsaw University of Technology, Noakowskiego 3, 00-664 Warsaw, Poland
b Institute of Physical Chemistry, Polish Academy of Sciences, Kasprzaka 44/52, 01-224 Warsaw, Poland
DOI: 10.1021/om5008117
First published online: 9 Sep 2014
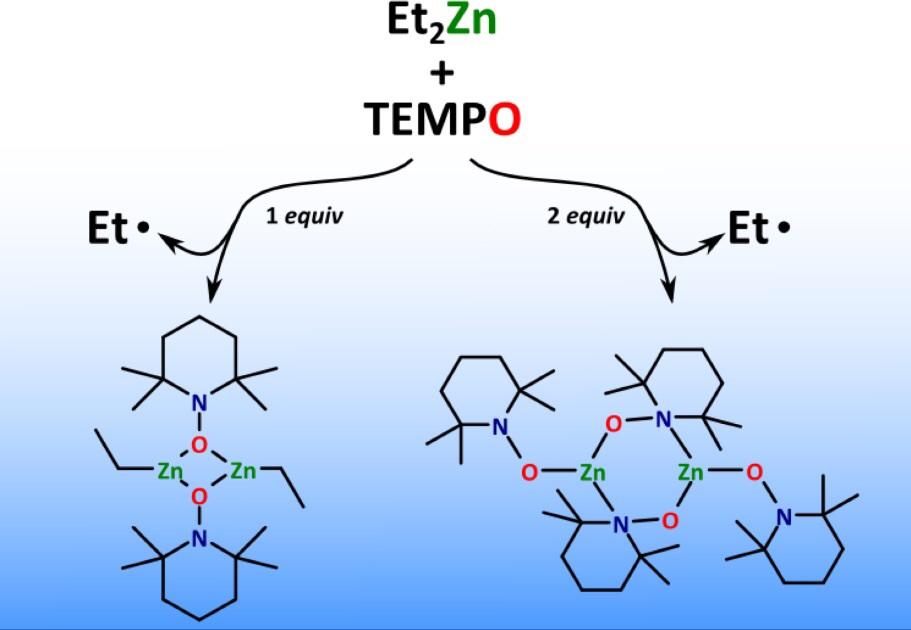
Reactions of diethylzinc with TEMPO were investigated. Dropwise addition of 1 equiv of TEMPO to Et2Zn at −10 °C leads to the nitroxide complex EtZn(TEMPO) in high yield, whereas upon addition of 2 equiv of TEMPO the corresponding homoleptic nitroxide compound Zn(TEMPO)2 is formed. Diffusion ordered NMR spectroscopy experiments revealed that both zinc nitroxide compounds exist in monomeric forms in solution, while single-crystal X-ray diffraction confirmed their dimeric structure in the solid state.