Kamil Sokołowski,a Wojciech Bury,b Iwona Justyniak,a Anna M. Cieślak,a Małgorzata Wolska,b Katarzyna Sołtys,a Igor Dzięcielewskic and Janusz Lewiński*ab
a Institute of Physical Chemistry, Polish Academy of Sciences, Kasprzaka 44/52, 01-224 Warsaw, Poland
b Faculty of Chemistry, Warsaw University of Technology, Noakowskiego 3, 00-664 Warsaw, Poland
c Institute of High Pressure Physics Unipress, Polish Academy of Sciences, Sokolowska 29/37, 01-142 Warsaw, Poland
DOI: 10.1039/C3CC41639A
Received 04 Mar 2013, Accepted 18 Apr 2013
First published online 18 Apr 2013
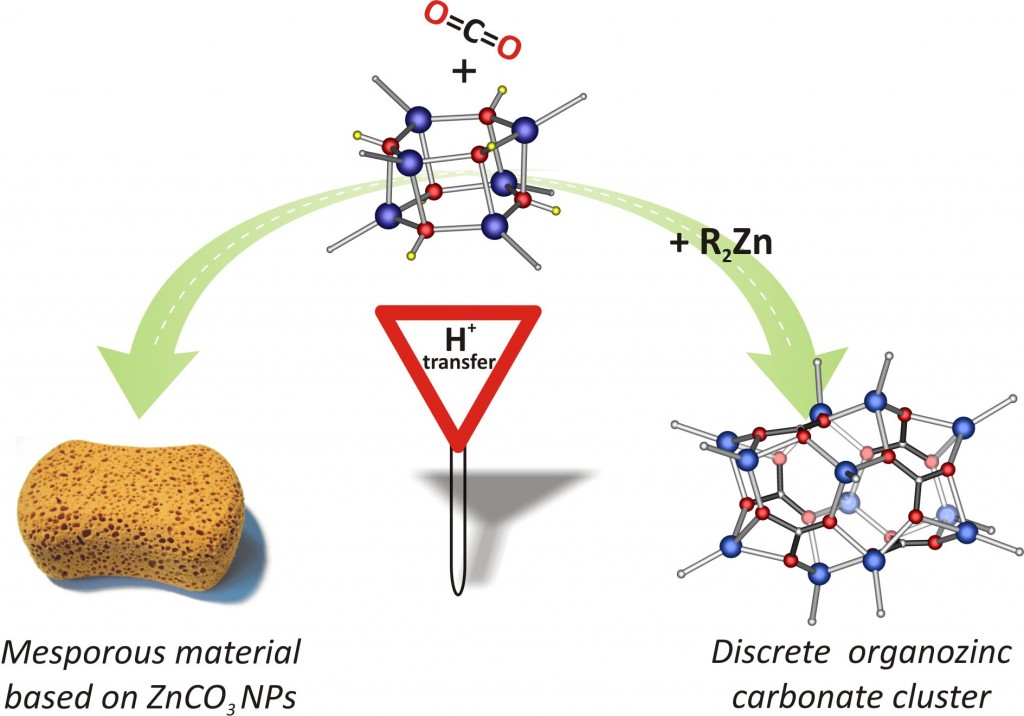
We report on the activation of CO2 by the well-defined alkylzinc hydroxide (tBuZnOH)6 in the absence and presence of tBu2Zn as an external proton acceptor. The slight modifications in reaction systems involving organozinc precursors enable control of the reaction products with high selectivity leading to the isolation of the mesoporous solid based on ZnCO3 nanoparticles or an unprecedented discrete alkylzinc carbonate [(tBuZn)2(μ5-CO3)]6 cluster with the Zn–C bond intact, respectively.