Catalytic Epoxidation of Enones Mediated by Zinc Alkylperoxide/tert-BuOOH Systems
Marcin Kubisiak,a Karolina Zelga,a Iwona Justyniak,b Ewa Tratkiewicz,a Tomasz Pietrzak,a Abdul R. Keeri,ac Zbigniew Ochal,a Larissa Hartenstein,d Peter W. Roeskyd and Janusz Lewiński*ab
a Faculty of Chemistry, Warsaw University of Technology, Noakowskiego 3, 00-664 Warsaw, Poland
b Institute of Physical Chemistry, Polish Academy of Sciences, Kasprzaka 44/52, 01-224 Warsaw, Poland
c University of Warsaw, Faculty of Chemistry, Pasteura 1, 02-093 Warsaw, Poland
d Institute for Inorganic Chemistry, Karlsruhe Institute of Technology, Engesserstr. 15, Geb. 30.45, 76131 Karlsruhe, Germany
DOI: 10.1021/om400830e
Received 13 Aug 2013, Accepted 17 Sep 2013
First published online 18 Sep 2013
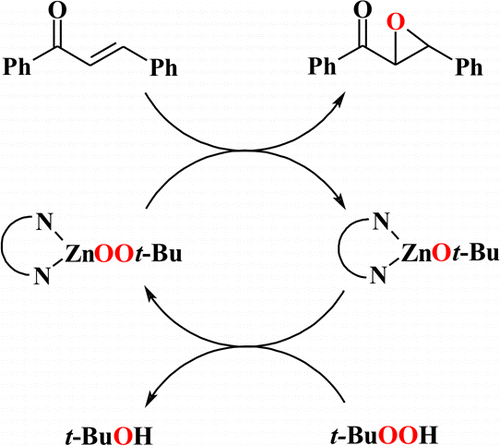
The epoxidation of enones by zinc alkylperoxides is a challenging task receiving considerable attention in contemporary research; however, until now no well-defined zinc alkylperoxide based systems have been described. Here, a new catalytic method of epoxidation of enones in the presence of zinc alkylperoxides supported by N,N-bidentate ligands and tert-butyl hydroperoxide is reported. A new dimeric zinc alkylperoxide complex supported by an aminotroponiminate ligand is also presented. The studied catalytic systems show high activity in the epoxidation of trans-chalcone, and in the case of a chiral catalyst with the (S,S)-N,N′-bis(1-phenylethyl)aminotroponiminate ligand a moderate enantioselectivity was achieved.